
Medication Benefit-Risk Calculator
How to Use This Tool
Answer these three key questions to understand if a medication's benefits outweigh its risks:
-
How severe is your condition?
Severe (10) → Mild (1)
-
How effective is treatment?
Highly effective (100%) → Ineffective (0%)
-
How likely are side effects?
Very common (20%) → Rare (0.1%)
This medication is not recommended
The benefits don't outweigh the risks for your condition.
Every time a doctor prescribes a pill, they’re making a silent calculation: Is this drug worth it? Not just for the disease it treats, but for the body it might harm. This isn’t guesswork. It’s a structured, evidence-based process called benefit-risk assessment-and it’s the backbone of every medication decision in modern medicine.
It’s Not About Eliminating Risk-It’s About Managing It
No medication is risk-free. Even aspirin can cause internal bleeding. Antibiotics can trigger life-threatening allergies. Chemo can destroy healthy cells along with cancer. But we don’t stop using these drugs. Why? Because the benefit-stopping a stroke, curing an infection, extending life-often far outweighs the danger. The U.S. Food and Drug Administration (FDA) made this principle official in 2013. They declared: a drug can only be approved if its benefits are expected to outweigh its risks. That’s not a suggestion. It’s the law. And it applies not just to new drugs, but to how doctors use them every day. Think of it like this: if you’re diagnosed with a rare, fatal disease and the only treatment has a 20% chance of serious liver damage, you might say yes. But if you’re healthy and told to take a daily pill to lower your slightly elevated blood pressure-with the same 20% risk-you’d probably say no. The same drug. Different context. That’s the heart of benefit-risk assessment.How Providers Actually Do the Math
Doctors don’t just wing it. They use a clear framework, even if they don’t say it out loud. Here’s how it breaks down:- What’s the condition? Is it life-threatening? Chronic? Mild? A heart attack demands aggressive treatment. A runny nose doesn’t.
- What are the alternatives? Are there safer drugs? Lifestyle changes? Surgery? If the only option is high-risk, the bar for benefit is lower.
- How well does it work? Is it a 70% cure rate? A 10% improvement in pain? Numbers matter. A drug that cuts cancer deaths by half is treated differently than one that eases occasional headaches.
- What are the side effects? Not just what they are, but how often and how bad. A 1% chance of a temporary rash? Acceptable. A 5% chance of permanent nerve damage? Not so much.
- What don’t we know? New drugs often lack long-term data. About 60% of drugs approved under fast-track programs have uncertain safety beyond a few years. That uncertainty gets weighed in.
Patients and Doctors Don’t Always See Eye to Eye
Here’s where things get messy. Patients don’t think like doctors. A 2023 survey by the Michael J. Fox Foundation found that 65% of Parkinson’s patients would accept a 20% risk of involuntary movements (dyskinesia) for a 30% improvement in mobility. Doctors estimated patients would only accept a 12% risk. Why the gap? Patients aren’t just thinking about longevity-they’re thinking about quality of life. Can they hug their grandkids? Walk to the mailbox? Eat without help? On the flip side, a Reddit thread from a pharmacist in 2023 described patients refusing ACE inhibitors for high blood pressure because they heard about a 0.1% chance of angioedema-a rare but scary swelling of the throat. Meanwhile, the drug reduces stroke risk by 25%. The math favors the drug. But fear doesn’t follow logic. This disconnect is real. A 2022 study showed patients with chronic conditions valued symptom relief over extended life by a 3-to-1 margin. Clinicians? They focused on survival. That’s why patient input is now built into FDA decisions. Since 2016, the agency has gathered feedback from over 1,200 rare disease patients. Their voices now shape drug labels and warnings.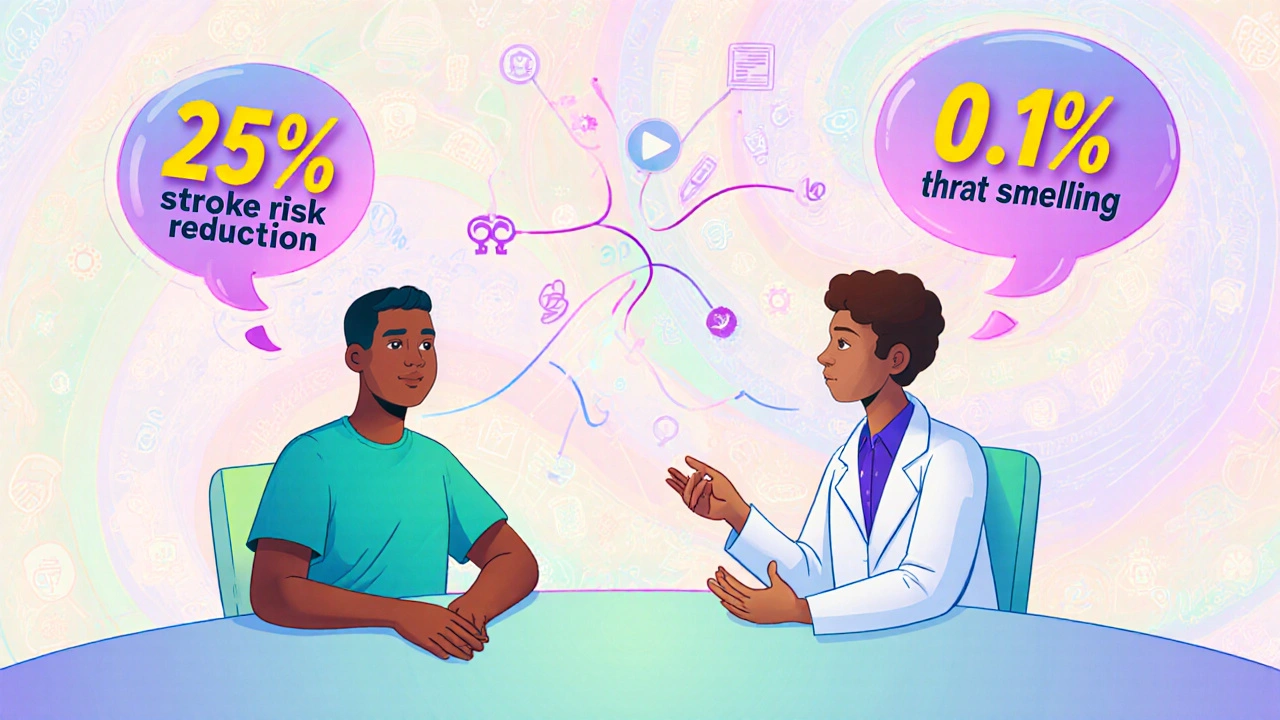
Why Some Drugs Get Rejected-Even When They Work
Not every effective drug gets approved. Sometimes, the risk is just too high for the benefit. In 2022, the FDA hesitated to approve new cardiovascular drugs for low-risk patients-even though they lowered cholesterol significantly. Why? Because they also increased the risk of dangerous bleeding. For someone with a 5% chance of a heart attack in 10 years, that trade-off didn’t add up. The benefit was too small. The risk was too real. A 2023 FDA report showed that 72% of drug rejections came down to an unclear or unfavorable benefit-risk balance. Often, it wasn’t that the drug didn’t work. It was that the side effects were too frequent, too severe, or too unpredictable for the condition being treated. This is why oncology drugs get more leeway. In 2022, 38% of all new drug approvals were for cancer. Why? Because the alternative is death. A drug that extends life by three months with severe fatigue? Many patients say yes. The same drug for mild arthritis? No chance.The Hidden Costs: Time, Money, and Trust
Benefit-risk decisions aren’t just clinical-they’re logistical and emotional. Primary care doctors spend 15 to 20 minutes per patient just explaining risks and benefits, according to the American Medical Association. That’s a huge chunk of a 10-minute visit. Many patients walk away confused. One 2023 study found only 35% understood what “10% risk” actually meant. Is that 1 in 10? 1 in 100? The difference matters. To fix this, the FDA launched patient decision aids-simple tools that show real numbers, visuals, and options. At Mayo Clinic and Johns Hopkins, these tools cut medication non-adherence by 22%. People stick with their meds when they truly understand why. And then there’s the money. Pharmaceutical companies spend an average of $150 million per drug on post-market safety studies. Complex safety programs (called REMS) can cost half a billion dollars a year. That’s not just bureaucracy-it’s protection. Every time a drug causes harm after approval, the system has to catch it. That’s why the global pharmacovigilance market is growing fast-projected to hit $14.2 billion by 2027.
The Future: Personalized Risk, Not One-Size-Fits-All
The next big shift? Making benefit-risk assessments personal. Right now, most decisions are based on averages: “Most people in this group get better, but some get sick.” But what if we knew your genes, your liver function, your diet, your sleep habits? What if we could predict whether *you* were likely to have side effects before you even took the pill? That’s not science fiction. The Precision Medicine Initiative predicts that by 2030, 70% of benefit-risk assessments will include individual data. AI tools are already helping companies like Roche spot safety signals in real-world data 30% faster. But there’s a big problem: most clinical trials still use mostly white participants. A 2023 study found that 75% of trial volunteers were White-even though minorities make up 40% of the U.S. population. That means the risks we calculate might not apply to everyone. Fixing this isn’t just fair-it’s necessary for accurate decisions.What This Means for You
If you’re taking a medication, ask:- What’s the chance this will actually help me?
- What are the most common side effects-and how bad are they?
- Is there a safer alternative?
- What happens if I don’t take it?
Why do some medications get approved even if they have serious side effects?
Medications with serious side effects are approved when the condition they treat is severe and has few or no alternatives. For example, cancer drugs often cause nausea, hair loss, or immune damage-but they can extend life by months or years. The FDA and doctors weigh whether the benefit-like survival or symptom relief-outweighs the harm. If the alternative is death or permanent disability, a high-risk drug may still be the best choice.
How do doctors know if a drug’s benefits outweigh its risks?
Doctors use data from clinical trials, real-world use, and guidelines from agencies like the FDA. They look at how often side effects happen, how severe they are, how well the drug works compared to other options, and whether the patient’s condition is life-threatening. They also consider what’s known and what’s uncertain-like long-term effects for new drugs. It’s not a simple formula, but a careful judgment based on evidence and experience.
Why do patients sometimes refuse medications that doctors recommend?
Patients often focus on the fear of side effects, even when they’re rare. For example, a 0.1% chance of throat swelling might sound terrifying-even though the drug cuts stroke risk by 25%. Patients also value quality of life more than doctors sometimes assume. Someone with chronic pain might prefer a drug that helps them walk again, even if it causes drowsiness. Communication gaps and misunderstanding of numbers play a big role too.
Are newer drugs riskier than older ones?
Newer drugs often have less long-term safety data, so their risks aren’t fully known. But they’re not necessarily riskier overall. Many are designed to be more targeted, with fewer side effects than older drugs. For example, newer diabetes medications cause less weight gain and low blood sugar than older ones. The FDA requires ongoing monitoring after approval, so hidden risks can show up later-but that’s part of the system, not a flaw.
Can I trust my doctor’s decision if they say a risky drug is right for me?
Yes, if they explain why. A good doctor doesn’t just say, “Take this.” They walk you through the numbers: “This drug gives you a 60% chance of improvement, but a 15% chance of serious side effects. The alternative is worsening symptoms over time.” If they’re transparent, use clear language, and respect your values, you can trust their judgment. If they dismiss your concerns or avoid details, it’s okay to ask for more info or a second opinion.
9 Comments
Robert Gilmore November 11, 2025 AT 18:48
I used to think doctors just handed out pills like candy until my mom got prescribed that high-risk blood thinner. Turns out they’re doing calculus in their head while you’re scrolling on your phone. The fact that they even consider your lifestyle, your fears, your grandkids-that’s not just medicine, that’s human.
Robert Gilmore November 13, 2025 AT 01:42
Actually, the FDA’s benefit-risk framework is rooted in utilitarian ethics, not clinical intuition. The 2013 policy formalized what pharmacoeconomists have been arguing since the 90s: marginal utility of survival must exceed marginal cost of adverse events. Most patients don’t grasp that a 25% stroke reduction isn’t ‘good enough’ if the drug increases intracranial hemorrhage by 1.2%. That’s why shared decision-making tools are essential-not just for compliance, but for ethical legitimacy.
Robert Gilmore November 14, 2025 AT 06:11
Here’s the uncomfortable truth: medicine isn’t science-it’s a negotiation between fear and hope… and the system is rigged. Pharma spends billions lobbying to get drugs approved under fast-track, then turns around and charges $2.1 million for a one-time cure… while the average patient can’t even afford the copay for the statin that might’ve prevented the whole mess. Who’s really benefiting? Not you. Not me. Definitely not the guy in rural Alabama waiting six months for a specialist.
Robert Gilmore November 15, 2025 AT 08:36
There is a critical error in the article’s use of the phrase ‘the same drug.’ This is misleading. Zolgensma is not the same drug as an ACE inhibitor. They are not interchangeable, nor are they comparable in mechanism, indication, or risk profile. Precision in language is not pedantry-it is the foundation of medical literacy. Please correct this.
Robert Gilmore November 16, 2025 AT 16:17
Most of this is just corporate spin. Doctors don't care about your quality of life. They care about hitting their KPIs and not getting sued. If you're not on 5 meds by 50, you're not being properly managed.
Robert Gilmore November 18, 2025 AT 08:40
My dad took that one blood pressure pill for 3 years. Started forgetting his own birthday. Stopped it. Now he remembers everything. Sometimes the risk isn’t worth the ‘benefit.’
Robert Gilmore November 19, 2025 AT 21:02
Back home in South Africa, we don’t have the luxury of ‘risk vs benefit’ debates. If you’re diabetic and you get insulin, you’re lucky. No one’s asking about long-term kidney damage because the alternative is dying in a week. The fact that we’re even having this conversation in the U.S. shows how far we’ve come… and how disconnected we’ve become from what real scarcity looks like.
Robert Gilmore November 21, 2025 AT 20:09
Wait-so if I’m a 65-year-old with mild hypertension and I refuse a drug because I’m scared of a 0.1% chance of throat swelling… am I being irrational? Or am I just prioritizing my peace of mind over a number on a chart? I don’t want to live longer if I have to live terrified. And why does that feel like a weakness? We need more stories like this-not just stats.
Robert Gilmore November 22, 2025 AT 22:31
They say ‘personalized medicine’ is the future… but what if it’s just a cover for selective data? You know they’re ignoring Black, Indigenous, and Asian populations in trials, right? That’s not science-it’s genocide by algorithm. And now they want to use AI to ‘predict your risk’ based on data that doesn’t even include you? I’m not taking anything until they fix that.